Glossary
21 CFR part 11
The technical and organizational requirements which need to be satisfied in order to be allowed to use electronic data and documentation in development, licensing, and production instead of paper are set down in Guideline 21 CFR part 11, initiated by the American authority, the FDA.
Adsorption
This refers to the accumulation of (amphiphilic) molecules on surfaces and interfaces of liquids and solids. This generally leads to a reduction in the surface and interfacial tension.
Billmeyer formula (IV value acc. to Billmeyer)
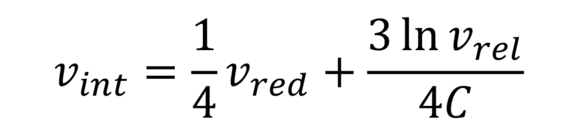
Serves the approximate calculation of intrinsic viscosity of polyesters and others. No additional polymer figures required.
Bubble pressure tensiometers
These determine the interfacial tension of liquids using the pressure in gas or air bubbles which are created in the liquid to be measured in a capillary with known dimensions. This method is not suited for determining interfacial tensions between liquid phases.
CMC measurement
This is the determining of a surfactant concentration at which the aggregation of the surfactant molecules begins to form micelles (CMC concentration). It is determined from the dependence of the concentration of the surfactant in the solution and the surface tension.
Diffusion coefficient
Characterizes the thermal diffusion of the molecules in the observed material. In tensiometry, the diffusion coefficient refers to the measured liquid.
Du-Noüy ring
This is a measuring body made of a platinum-iridium alloy to determine surface/interfacial tension from the tensile force of an attacking lamella produced by this body using a ring/plate tensiometer.
Dynamic interfacial/surface tension
This is the measurable reduction in interfacial/surface tension due to the adsorption of surface active substances.
Dynamic measuring methods (tensiometry)
These determine the surface and interfacial tension which is dependent on the surface age.
Dynamic viscosity
This is the viscosity coefficient material to shear flows between transverse stress τ velocity gradient D in τ = η D and has the mPas unit (previously Centipoise, cP).
Emulsion
This is a disperse system consisting of water or an aqueous solution and an organic liquid immiscible with water. The emulsified or inner phase is present in the form of small droplets. These are surrounded by the coherent or outer phase.
FDA
Short for “United States Food and Drug Administration“. It prescribes binding guidelines for the development and production of pharmaceutical products with international validity.
Glass viscometer
Viscometers made from glass, with different designs, standardized in ISO 3105. The most commonly used for automatic measurements is the Ubbelohde version with ventilation pipe.
GLP
Short for “Good Laboratory Practice“. Specifications initiated by the American authority, the FDA, for laboratories and producers (e.g. of pharmaceuticals) regarding how tests and measurements are to be cleanly planned, performed, and monitored. The guidelines have a legal character in many countries.
Interfacial energy
This is the sum of free energy of all the molecules found in the interface between different materials. The interface between a liquid and a gas is called the surface and the corresponding energy from this space is the surface energy.
Interfacial tension
This is the work that must be done to increase the interface of the liquid by one surface area unit (1 m2). This is equivalent to the specific interfacial energy.
Hagen-Poiseull‘s Law (fundamental equation of capillary viscometry)
Viscometry in capillary viscometers is based on this. If the differential pressure is generated by a height difference in front of and behind the capillaries, then νkin = k x t (k: capillary constant, t: measured run of a defined liquid volume) applies. With very short times, the non-dissipated kinetic energy must be taken into consideration (kinetic energy/Hagenbach correction).
Huggins formula (IV value according to Huggins)
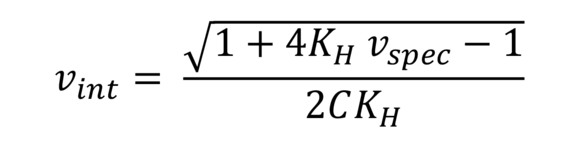
Serves the approximate calculation of intrinsic viscosity of, for example, polystyrenes and other polymers. KH is an additional polymer-dependent constant
Inherent viscosity (logarithmic viscosity number)
This is the natural logarithm of the relative viscosity in relation to the concentration C of the separated material νred = ln νrel / C
Unit: cm3/g = 100 dl/g
Intrinsic viscosity (limiting viscosity number, Staudinger Index, IV value)
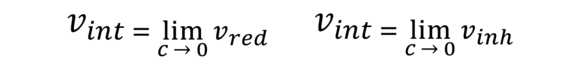
This is the limiting value of the reduced/inherent viscosity for the case of infinitely strongly diluted solvents at dissipating transverse stresses. It is determined by measuring the νred as a function of the concentration and extrapolation on C = 0. For many polymers, there are approximation conditions based on the measurement of only one concentration usually specified in standards.
Kinematic viscosity
Describes the quotient of the dynamic viscosity by the density: νkin = η / ρ has the unit mm2/s (formerly: centistokes, cst).
Kinetic energy correction (Hagenbach correction)
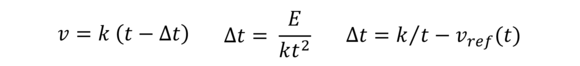
With short flow times, this is a correction necessary to be made to Hagen-Poiseull‘s Law and takes into consideration the kinetic energy in a capillary viscometer which is not converted into frictional heat.
On the left: Corrected viscosity, In the middle: Correction factor acc. to Hagenbach, On the right: Correction factor ISO 1628/6
Contact angle/Contact angle measurement
This characterizes the wetting properties of liquids on solid bodies. The contact angle can be determined by measuring individual drops on a solid or from the forces required to move a solid in contact with a liquid lamella. The contact angle is used to calculate the surface energy.
Correction acc. to Harkins and Jordan
Harkins and Jordan generated tables for the correction of the ring-measured surface and interfacial tension. These values are standardized
Correction acc. to Zuidema and Waters
Zuidema and Waters generated a polynomial for the correction of the ring-measured surface and interfacial tension.
Critical Micelle Concentration (CMC)
At this concentration, surfactant solutions suddenly change their physical properties. The reason for this is the formation of organized aggregates (micelles) of the surfactant molecules when the critical micelle concentration is exceeded. The structure of the micelles is ependent on the character of the solvent and the structure of the surfactant molecules.
K-value (acc. to Fikentscher)
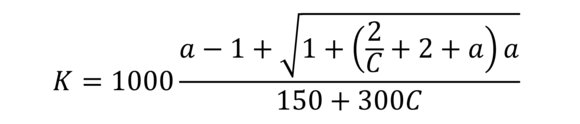
A relative measurement for the molar mass traditionally used for PVC and PVA with a = 1,5 logνrel
LIMS
Abbreviation for Laboratory Information Management System. This describes a system for the control and management of laboratory data, which is determined by various measuring devices.
Mark-Houwink formula
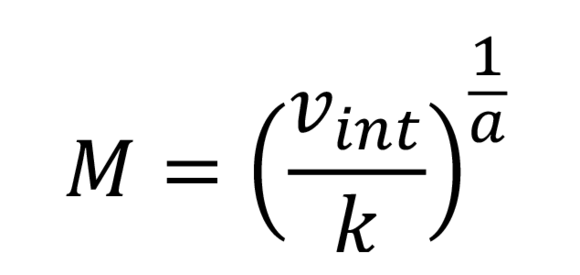
This provides the relation between medium molar mass (weight means) of the dissolved polymer chains and the intrinsic viscosity. For the absolute molar mass, the proportional constant K and the exponent a need to be entered. These depend on the polymer and the solvent, and can be found in the references.
Martin formula (IV value acc. to Martin)
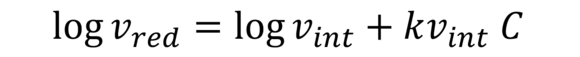
This serves the approximate calculation of intrinsic viscosity of, for example, cellulose and other polymers. K is an additional constant dependent on the polymer.
Surface age
The age of a surface since its formation. With the bubble pressure tensiometer, the surface age is the period from the beginning of bubble formation to the hemispherical shape of the bubble. With the drop volume tensiometer, this is the period between the formation and the separation of a drop.
Surface tension
This is the interfacial tension between a liquid and any gas.
Peltier thermostating
This is an alternative to conventional thermostating which is based on the Peltier effect. Here, two semi-conductors with different conduction bands are brought into contact with each other. If electricity is conducted through this array, electrons will be pumped on one side to a higher energy level. The heat energy extracted during this brings about a cool-down. On the other side, the electrons fall back down to the starting level and release heat again. By reversing the direction of electric current, both cooling and heating are possible. Peltier elements only operate electrically, that is, there are no moving components, gases, or liquids. The limited degree of efficiency has proven itself to be disadvantageous in a number of concepts.
Plate method acc. to Wilhelmy
This is used to measure the surface tension with the aid of a standardized plate with a ring/plate tensiometer. The plate is moved towards the surface until the meniscus connects with it. The surface tension is calculated from the resulting force. Due to uncertain wetting behavior, the plate can only be used for interfaces under certain limitations.
Reduced viscosity (viscosity number)
This is the specific viscosity in relation to the concentration C of the separated material: νred = : νsp / C
Unit: cm3/g = 0.01 dl/g
Relative viscosity
This is the ratio of the dynamic viscosity η of the solvent to that of the solvent η0. In the case of strongly diluted solvents, this corresponds almost to the ratio of the kinematic viscosities: νrel ≈ η / ηs ≈ ν / νs
Ring method acc. to Du-Noüy
This is used to measure the surface/interfacial tension with the aid of a standardized ring with a ring/plate tensiometer. The ring is submerged in the liquid and then extracted. A liquid lamella forms during this, which is stretched to the maximum. The surface and interfacial tension is calculated from the resulting force. Then, the determined value still needs to be corrected.
Ring/Plate tensiometers
These measure the force with which a lamella attacks a Du-Noüy ring or Wilhelmy plate. This is used to calculate the surface/interfacial tension between liquid phases.
Schulz-Blaschke (IV value acc. to Schulz-Blaschke)
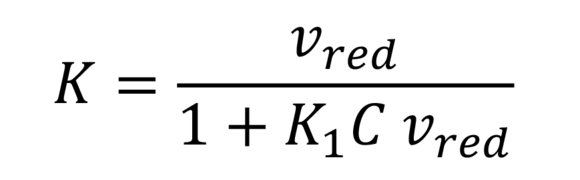
Serves the approximate calculation of intrinsic viscosity of cellulose, polyolefins, and other polymers. K1 is an additional constant dependent on the polymer.
Solomon-Ciuta formula (Solomon-Ciuta)
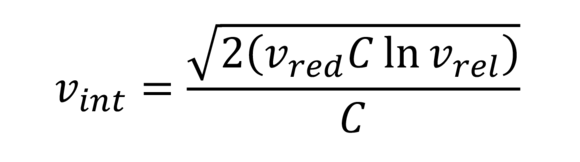
This serves the approximate calculation of intrinsic viscosity of PMMA and other polymers. No additional polymer figures required.
Specific viscosity (relative viscosity increase)
This is the relative viscosity minus one: νsp = νrel -1
Suspension
This is a disperse system, that is, a fine, but not molecular distribution of a solid body in a liquid.
Surfactant
This is a surface-active substance. The prerequisite for this activity is an asymmetric structure of the surfactant molecules, consisting of a hydrophobic (water-repelling) part and a hydrophilic (water-soluble) part. The interfacial tension is reduced by the adsorption of these molecules at the interface.
Drop volume tensiometers
These determine the surface/interfacial tension of liquids at the moment of drop separation, from the volume of the drop that is formed in air or in an indissoluble phase. The densities of the phases concerned must be known in order to determine the surface/interfacial tension from the drop volume measured.
Viscosity
This is the measure for how fluidly a liquid flows. The greater the viscosity, the thicker the liquid.
Viscosity index (for mineral oil products)
This is calculated according to ISO 2909 and ASTM D 2270 from the viscosities measured at the two different temperatures 40 and 100 °C. It is a measure of the temperature behavior of different oils. The higher the viscosity index of an oil, the less its viscosity changes at different temperatures.
Wilhelmy plate
Standardized plate, usually made of a platinum alloy. It is used to measure the surface tension with ring/plate tensiometers. The plate material has been chosen so that the contact angle with the measuring liquid can equal zero. Optimum cleaning, e.g. through annealing, is preconditioned.




